A review was recented offered in Current Opinions:
Treating Obstructive Sleep Apnea With Hypoglossal Nerve Stimulation
Arie Oliven
Posted: 10/18/2011; Curr Opin Pulm Med. 2011;17(6):419-424. © 2011 Lippincott Williams & Wilkins
Abstract and Introduction
Abstract
Purpose of review Obstructive sleep apnea (OSA) is a common disorder characterized by recurrent pharyngeal collapse secondary to sleep-induced hypotonia of peri-pharyngeal structures. Therapy for OSA is sometimes poorly tolerated and not always effective. The current study reviews a new treatment modality, hypoglossus stimulation, recently evaluated by multiple physiological studies and currently assessed by several clinical studies.
Recent findings A phase-I, implantable hypoglossus nerve stimulation multicenter study was published in 2001. Significant reduction in apnea–hypopnea index (AHI) was reported in seven of the eight implanted OSA patients, but technical faults precluded prolonged follow-up. Over the past 2 years, three new hypoglossus nerve stimulation systems have been evaluated in more than 60 OSA patients. In adequately selected patients, a more than 50% reduction in AHI was observed. Usually, a decrease in OSA severity from moderate–severe to mild–minimal can be achieved.
Summary Ongoing research, including recent initiation of a large multicenter phase-III study, suggests that hypoglossus nerve stimulators are likely to be available as a new treatment modality within a few years. Additional data are needed to define which OSA patients are most likely to benefit from hypoglossus nerve stimulation. Continuous refinement of electrodes design is likely to improve stimulation efficacy in coming years.
Introduction
Obstructive sleep apnea (OSA) is a common disorder characterized by recurrent episodes of upper airway obstruction during sleep.[1,2] In addition, OSA has been shown to increase the risk of hypertension and metabolic disorders, and consequently cardiovascular morbidity and mortality.[3–7] Although numerous methods have been used to restore upper airway patency during sleep, no single treatment modality has been shown to provide relief to all patients with this disorder, as all are only partially effective and/or may be poorly tolerated: continuous positive airway pressure (CPAP) has been the mainstay of treatment since the early 1980s, but incomplete compliance persists despite continuous technical improvements. A large number of oral appliances and operative solutions have been designed, but at times only partial improvement can be achieved.[8–11] Therefore, a new approach to the treatment of OSA is most desirable, preferentially an approach that will address the primary cause of pharyngeal obstruction in OSA, namely, the sleep-related decrease in pharyngeal dilating forces.
Electrical Stimulation of Upper Airway Dilator Muscles: Rationale
In addition to the many anatomical abnormalities implicated in the pathogenesis of OSA, the role of neuromuscular mechanisms is clear, as apneas occur only during sleep, when muscular activity wanes.[12,13•] Consequently, the importance of upper airway dilator muscles in maintaining pharyngeal stability has been long recognized. A progressive diminution in the level of electrical activity of upper airway dilator muscles with the transition from wakefulness to sleep has been well documented. This decrease in activity is associated with pharyngeal narrowing and increased upper airway resistance, and frank collapse in OSA patients.[13•] Accordingly, an approach directed to increase dilator muscle tone to a level sufficient to maintain pharyngeal patency during wakefulness is expected to restore airflow during sleep.
Experimental Animal Models
Functional electrical stimulation of striated muscles provides an important tool to assess their mechanical effect and has been largely employed since the early 1980s to assess the effects of pharyngeal dilator muscles on the upper airway. Virtually all animal studies have confirmed that stimulation of dilator muscles enlarges and stabilizes the pharynx. Results obtained in anesthetized animals need to be interpreted cautiously when applied to humans, as upper airway anatomy differs among mammals in several important aspects, and none of the animal models studied are prone to developing sleep apnea. Keeping these reservations in mind, the following are some of the animal studies that provided findings relevant to therapeutic dilator muscle stimulation: stimulation of hypoglossus nerve fibers that innervate the tongue appears to produce equal or larger mechanical effects compared to other cranial nerves that innervate pharyngeal and palatal muscles;[14] stimulation of the whole hypoglossus nerve, which co-activates both protrusor and retractor muscles of the tongue, enlarges the pharynx to a similar degree as selective stimulation of the medial branch of the hypoglossus nerve, which preferentially contracts tongue protrusors;[15] unilateral stimulation of the hypoglossus nerve produces responses comparable to bilateral stimulation in rabbits, even with sub-maximal stimulation intensities;[16] direct stimulation of the genioglossus muscle produces similar results to those obtained by stimulation of the hypoglossus nerve, suggesting that stimulation of the genioglossus muscle can predict responses to hypoglossus nerve stimulation;[17] comparison of the effects of several dilator muscles indicates that the mechanical response to genioglossus muscle stimulation is significantly larger than that of stimulation of other muscles, and is independent of head position[18] genioglossus muscle contraction can also ameliorate partial or complete pharyngeal obstruction;[19] in dogs implanted with hypoglossus nerve stimulators (HGNSs), mechanical efficacy is maintained despite chronic excitation over months without any sign of damage to the hypoglossus nerve;[20] and finally, specially designed electrodes may enable selective stimulation of specific fiber bundles within the hypoglossus nerve.[21]
Initial Dilator Muscle Stimulation Attempts in Humans
Attempts to stimulate upper airway dilating muscles in OSA patients for therapeutic purpose have been undertaken ever since the physiological importance of these muscles' action began to be appreciated. Although anecdotal reports describing dilator muscle stimulation with intramuscular electrodes date back to the late 1970s,[22] the first published trial reported a significant reduction in apneas with sub-mental electrical stimulation.[23] However, sub-mental stimulation causes contraction of the plathysma, but does not move the tongue even with strong, painful stimulation,[24] and the original findings could not be duplicated in other centers.[22,25,26] Even in patients with OSA, this technique produced only small and inconsistent improvement in pharyngeal patency. Similarly, fine-wire electrodes placed under computed tomography (CT) guidance near an internal branch of the hypoglossus nerve[26] and stimulation of the genioglossus muscle with surface or intramuscular electrodes[27–29] were not found useful for clinical purposes: although improvements in flow mechanics could be demonstrated, adequate flow at ambient pressure was observed in only a few OSA patients, and the stimulation was found to result in arousal after only a few breaths.
Physiological Studies in Humans
Genioglossus muscle stimulation during sleep that results in genioglossus muscle contraction reduces the critical occlusion pressure of the pharynx (Pcrit).[29] Recent studies conducted under propofol anesthesia ('pharmacological sleep') provided additional physiological findings relevant to OSA: genioglossus muscle stimulation was shown to lower Pcrit when the primary site of collapse was at the level of the velopharynx as well.[30] Genioglossus muscle contraction appears to improve pharyngeal stability by reducing extrapharyngeal pressure, rather than by lowering pharyngeal compliance.[31•] Interestingly, advancement of the tongue is not mandatory, as genioglossus muscle stimulation often also enlarges the velopharynx in the lateral direction.[32] However, it is important to recruit the appropriate tongue fibers: the fan-like orientation of the genioglossus muscle fibers produces both tongue depressant and protrusive effects, and only the latter improves flow-mechanics.[33•] Therefore, more targeted stimulation would be expected to optimize the mechanical response. Independently of the site of stimulation, large differences in the response to stimulation were observed between OSA patients.[29,30,33•] These differences did not correlate with the anthropometric, polysomnographic, or cephalometric characteristics of these OSA patients. However, patients with a relatively larger tongue tended to have a larger response.[33•] Changes in length-tension relationships may affect the mechanical response to genioglossus muscle contraction, but under physiological conditions this effect appears to be minimal.[34] Also, co-activation of tongue retractors produced a similar response to that of isolated genioglossus muscle stimulation.[35] All of these physiological human studies, while confirming the feasibility and mechanical efficacy of tongue muscle stimulation, also suggested that only neurostimulation of the pure motor hypoglossus nerve, which avoids the sensation of the stimulation by sensory lingual nerve fibers, has a potential to become clinically relevant as a new treatment modality for OSA.
Hypoglossus Nerve Stimulation in Obstructive Sleep Apnea Patients
The major breakthrough for this new OSA treatment modality occurred when investigators from Johns Hopkins University initiated a series of well designed studies leading ultimately to an implantable hypoglossus nerve stimulation system. In a preliminary acute study, five OSA patients were implanted with hypoglossus nerve cuff electrodes and were transferred, after arousal from anesthesia, to the sleep laboratory. Electrodes were activated by an external stimulator, and measurements performed during sleep showed an improvement in inspiratory airflow ranging between 50 and 200 ml/s, without arousal.[36] The success of this preliminary study led to approval and initiation of a phase I, hypoglossus nerve stimulation, multicenter feasibility study undertaken to investigate the efficacy of a fully implantable hypoglossus nerve stimulation system. The system (Inspire I; Medtronic, Minneapolis, Minnesota, USA) consisted of three components: an implantable pacemaker-like pulse generator, a tri-polar, half-cuff peripheral nerve-stimulation electrode, and a pressure sensor designed to recognize intrathoracic pressure deflection and initiate hypoglossus nerve stimulation during inspiration. Limiting stimulation to inspiration was physiologically logical and considered necessary to prevent neuromuscular fatigue. The system was implanted under general anesthesia through three surgical incisions: the stimulation electrode was placed on the peripheral main branch of the hypoglossus nerve close to its entrance into the tongue muscle. The pulse generator was placed subcutaneously into an infraclavicular pocket. The pressure transducer was inserted into a hole drilled through the manubrium sterni, thereby allowing the measurement of intrathoracic pressure. The system contained a microprocessor that could be reprogrammed via transcutaneous instructions, enabling the physician to adjust stimulus frequency, duration, and amplitude. A remote control unit allowed the patient to turn the stimulator on at bedtime, and a preset delay enabled patients to fall asleep before the start of electrical stimulation.
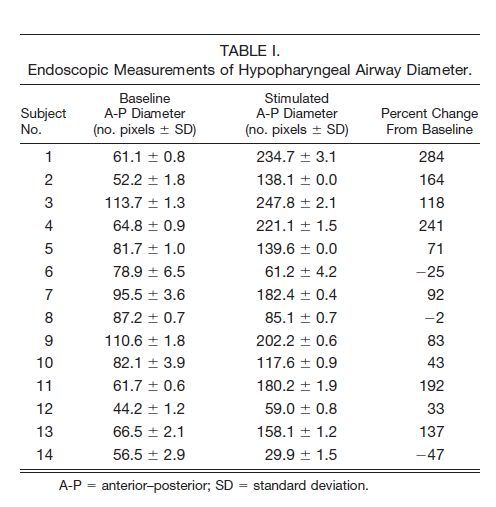
The results of the study were published in 2001[36] and in several later reviews, including a recent comprehensive description of the same study.[37•] A substantial reduction in the apnea–hypopnea index (AHI) was observed in all but one of the eight patients implanted. The improvement was already appreciable in the first postimplantation sleep study performed after 1 month, and the same response was maintained over a follow-up period of 6 months. On average, the AHI in the seven successfully implanted OSA patients decreased from 53.4±22.2 to 21.4±9.8 events/h (mean±SD) and fell as low as 13.3±6.6 events/h if nonsynchronized stimulations were excluded. Unfortunately, technical faults that included lead rupture and sensor malfunctioning due to cardiac artifacts precluded prolonged assessment.[37•] Nevertheless, all patients tolerated the long-term stimulation and did not experience any adverse effects, and the three patients who remained free from stimulator malfunction continued to use the device as primary treatment until the lifetime of the internal power supply was reached.[37•]
Recent Ongoing Phase II Hypoglossus Nerve Stimulation Studies
Despite the positive results of the phase I feasibility study, it took more than 10 years before improved stimulation systems were developed and new studies were initiated. In 2009, two investigator groups started multisite studies, and a third group joined a year later. The new systems remained basically similar, with the exception of the respiratory sensor, which no longer depends on intrathoracic pressure (Fig. 1). At the time of this writing, interim results of these ongoing studies have been presented at the 2011 American Thoracic Society International Conference in Denver, Colorado. More than 60 patients had received the new devices in the USA, Europe, Israel, and Australia. Eastwood et al.[38••] reported their findings in 21 individuals (14 men) implanted with a novel HGNS (Apnex Medical Inc., St Paul, Minnesota, USA) and followed for 6 months. These investigators selected patients with predominantly obstructive hypopneas and used sensors that identified inspiration by changes in thoracic impedance. Seventy five per cent of their patients experienced greater than 50% decrease in AHI; the mean_SD AHI decreased from 43.1±17.5 to 16.3±9.1 events/h, associated with significant reduction in desaturation events and improvement in Epworth Sleepiness Scale and Functional Outcomes of Sleep Questionnaire scores. Two adverse events (stimulator pocket infection and electrode dislodgement) required surgical intervention but resolved without sequelae. Well synchronized stimulations increased inspiratory flow by 15.2±2.4 l/min.[39] Badr et al.[40••] reported their results with the Inspire system (Inspire Medical Systems, Minneapolis, Minnesota, USA), a modified and improved version of the original phase I system, now using a sensor that monitors respiratory movements. The first 22 patients enrolled had a wide range of BMI and AHI, and only eight of them were considered responders. Based on findings obtained in this first group, new selection criteria and electrode placement site were introduced. In a second group of nine individuals, all but one showed substantial improvement, with a reduction in AHI for this group from 39±9 to 12±13 events/h. The new Inspire device provided long-term safety, stability, and functional reliability over a follow-up period of more than 2 years. These results prompted a phase III study: the Stimulation Therapy for Apnea Reduction, or STAR trial. Rodenstein et al.[41••] reported their results on 11 OSA patients implanted with an original system (aura6000; ImThera Medical, SanDiego,California,USA).This device uses a silicone cuff electrode housing six independent contacts surrounding the hypoglossus nerve. This approach, although requiring elaborate titration of stimulation during wakefulness and sleep, enables a continuous ('tonic') hypoglossus nerve stimulation without the need to synchronize stimulation with the respiratory cycle. Partial unilateral tongue paresis lasting 2–3 months occurred in two participants. AHI decreased significantly from 47.4±16.9 to 19.4±12.6 events/h, associated with a corresponding decrease in desaturations.

Figure 1. Schematic presentation of the implantable hypoglossus nerve stimulation system, designed to contract the tongue muscles during inspiration
Tetanic stimulations to the hypoglossus nerve lasting 1–2 s are applied by a pacemaker-like device, triggered by a respiratory sensor so as to be synchronized to inspiration
Prospective Developments and Future Research
Although the results of phase I and II studies are encouraging, they also suggest that hypoglossus nerve stimulation usually produces only a partial response. Consequently, this modality may be applicable to only certain OSA patients and appropriate selection criteria must be developed. Furthermore, modification of the electrodes to allow more complex stimulation algorithms is being pursued,[21,41••] and it remains to be seen whether this extension of the technique will improve targeted stimulation and mechanical efficacy. Although the effect of hypoglossus nerve stimulation during sleep appears to be primarily mediated through a mechanical process,[39] chronic stimulation may affect both control of genioglossus respiratory activity and histochemical and functional changes in the activated muscle fibers. The magnitude and clinical implications of these changes remain to be evaluated.
Conclusion
To the present time, approximately 70 OSA patients have been treated with unilateral hypoglossus nerve stimulators. Overall, the results can be considered promising: substantial reductions in AHI, associated with improved oxygenation, and of subjective parameters have been demonstrated in adequately selected patients. Although general anesthesia is required, the operative procedures are performed subcutaneously, and all patients are mobile within a few hours after implantation. Few major adverse events requiring removal of the implants occurred, and none resulted in permanent morbidity. The implanted devices were well tolerated by the patients, resulting in good adherence to regular use of the stimulator on a nightly basis by responders, suggesting the possibility of a clear advantage compared to CPAP in some patients. The convenience of use may compensate for the cost and invasiveness of the procedure, thus providing an effective treatment modality for the population of OSA patients who cannot tolerate CPAP. However, in contrast to CPAP, hypoglossus nerve stimulation in its current unilateral version provides only a partial reduction in AHI in most patients. Also, an acceptable trade-off may exist for those patients able to use CPAP for only part of the night: partial reduction in AHI for an entire night using hypoglossus nerve stimulation vs. normalization of AHI for only part of the night on CPAP. Nevertheless, many of the most severe OSA patients (i.e. those with AHI >50) are probably inadequate candidates as hypoglossus nerve stimulation will most likely not produce a fall in AHI to below 20/h. Additional parameters that may predict response and could then improve patient selection need to be determined in future studies. In addition, once long-term safety of unilateral hypoglossus nerve stimulation is confirmed, further development of bilateral HGNS technology will likely improve the mechanical efficiency of this technique and allow extension of this treatment modality to a greater range of patients.
Sidebar
Key Points
•Obstructive sleep apnea is largely due to sleep-related decrease in pharyngeal dilator muscle tone.
•Therefore, electrical stimulation of dilator muscles to restore their tone addresses directly the pathophysiological cause of obstruction.
•Hypoglossus nerve and direct genioglossus muscle stimulation have been shown in multiple animal and human studies to improve pharyngeal patency.
•Ongoing phase II and III studies with fully implantable hypoglossus nerve stimulation systems have proven safe, convenient, and successful in selected patients, suggesting that this new treatment modality may be available for clinical use in a few years.
References
1.Yaggi HK, Strohl KP. Adult obstructive sleep apnea/hypopnea syndrome: definitions, risk factors, and pathogenesis. ClinChestMed 2010; 31:179–186.
2.Kapur VK. Obstructive sleep apnea: diagnosis, epidemiology, and economics. Respir Care 2010; 55:1155–1167.
3.Lloberes P, Lozano L, Sampol G, et al. Obstructive sleep apnoea and 24-h blood pressure in patients with resistant hypertension. J Sleep Res 2010; 19:597–602.
4.Angelico F, del Ben M, Augelletti T, et al. Obstructive sleep apnoea syndrome and the metabolic syndrome in an internal medicine setting. Eur J Intern Med 2010; 21:191–195.
5.Green DE, Schulman DA. Obstructive sleep apnea and cardiovascular disease. Curr Treat Options Cardiovasc Med 2010; 12:342–354.
6.Ozol D, Turkay C, Kasapoglu B, et al. Relationship between components of metabolic syndrome and polysomnographic findings in obstructive sleep apnea. Metab Syndr Relat Disord 2011; 9:13–18.
7.Baguet JP, Barone-Rochette G, Lévy P, et al. Left ventricular diastolic dysfunction is linked to severity of obstructive sleep apnoea. Eur Respir J 2010; 36:1323–1329.
8.Eastwood PR, Malhotra A, Palmer LJ, et al. Obstructive sleep apnoea: from pathogenesis to treatment – current controversies and future directions. Respirology 2010; 15:587–595.
9.Holty JE, Guilleminault C. Maxillomandibular advancement for the treatment of obstructive sleep apnea: a systematic review and meta-analysis. Sleep Med Rev 2010; 14:287–297.
10.Kezirian EJ, Malhotra A, Goldberg AN, White DP. Changes in obstructive sleep apnea severity, biomarkers, and quality of life after multilevel surgery. Laryngoscope 2010; 120:1481–1488.
11.Caples SM, Rowley JA, Prinsell JR, et al. Surgical modifications of the upper airway for obstructive sleep apnea in adults: a systematic review and meta-analysis. Sleep 2010; 33:1396–1407.
12.Vos WG, De Backer WA, Verhulst SL. Correlation between the severity of sleep apnea and upper airway morphology in pediatric and adult patients. Curr Opin Allergy Clin Immunol 2010; 10:26–33.
13.Dempsey JA, Veasey SC, Morgan BJ, O'Donnell CP. Pathophysiology of sleep apnea. Physiol Rev 2010; 90:47–112.
• A recent and most comprehensive analysis of the pathophysiology of sleep apnea.
14.Kuna ST. Regional effects of selective pharyngeal muscle activation on airway shape. Am J Respir Crit Care Med 2004; 169:1063–1069.
15.Fregosi RF. Influence of tongue muscle contraction and dynamic airway pressure on velopharyngeal volume in the rat. J Appl Physiol 2008; 104:682–693.
16.Bellemare F, Pecchiari M, Bandini M, et al. Reversibility of airflow obstruction by hypoglossus nerve stimulation in anesthetized rabbits. Am J Respir Crit Care Med 2005; 172:606–612.
17.Oliven A, Schnall R, Odeh M. Improved upper airway patency elicited by electrical stimulation of the hypoglossus nerves. Respiration 1996; 63:213–216.
18.Odeh M, Schnall R, Gavriely N, Oliven A. Dependency of upper airway patency on head position: the effect of muscle contraction. Respir Physiol 1995; 100:239–244.
19.Bishara S, Odeh M, Schnall R, Oliven A. Electrically activated dilator muscles reduce pharyngeal resistance in anesthetized dogs with upper airway obstruction. Eur Respir J 1995; 8:1537–1542.
20.Goding GS, Eisele DW, Testerman R, et al. Relief of upper airway obstruction with hypoglossal nerve stimulation in the canine. Laryngoscope 1998; 108:162–169.
21.Yoo PB, Durand DM. Effects of selective hypoglossal nerve stimulation on canine upper airway mechanics. J Appl Physiol 2005; 99:937–943.
22.Guilleminault CN, Powell N, Bowman B, Stoohs R. The effect of electrical stimulation on obstructive sleep apnea syndrome. Chest 1995; 107:67–73.
23.Miki H, Hida W, Chonan T, et al. Effects of submental electrical stimulation during sleep on upper airway patency in patients with obstructive sleep apnea. Am Rev Respir Dis 1989; 140:1279–1284.
24.Schnall R, Pillar G, Kelsen SG, Oliven A. Dilatory effects of upper airway muscle contraction induced by electrical stimulation in awake humans. J Appl Physiol 1995; 78:1950–1956.
25.Edmonds LC, Daniels BK, Stanson AW, et al. The effects of transcutaneous electrical stimulation during wakefulness and sleep in patients with obstructive sleep apnea. Am J Respir Dis 1992; 146:1030–1036.
26.Decker MJ, Haaga J, Arnold JL, et al. Functional electrical stimulation and respiration during sleep. J Appl Physiol 1993; 75:1053–1061.
27.Oliven A, Schnall RP, Pillar G, et al. Sublingual electrical stimulation of the tongue during wakefulness and sleep. Respir Physiol 2001; 127:217–226.
28.Schwartz AR, Eisele DW, Hari A, et al. Electrical stimulation of the lingual musculature in obstructive sleep apnea. J Appl Physiol 1996; 81:643–652.
29.Oliven A, O'Hearn D, Boudewyns A, et al. Upper airway response to electrical stimulation of the genioglossus in obstructive sleep apnea. J Appl Physiol 2003; 95:2023–2029.
30.Oliven A, Tov N, Geitini L, et al. Effect of genioglossus contraction on pharyngeal lumen and airflow in sleep apnoea patients. Eur Respir J 2007; 30:1–11.
31.Oliven A, Kaufman E, Kaynan R, et al. Mechanical parameters determining pharyngeal collapsibility in patients with sleep apnea. J Appl Physiol 2010; 109:1037–1044.
• A physiological study that evaluated the effect of genioglossus stimulation on mechanical parameters of the 'tube law'. For readers interested in basic-science aspects of upper airway mechanics.
32.Dotan Y, Golibroda T, Netzer A, Oliven A. Interaction between tongue movement and velopharyngeal size and stability during genioglossus stimulation in patients with obstructive sleep apnea. Am J Respir Crit Care Med 2011; 183:A2725.
33.Dotan Y, Golibroda T, Oliven R, et al. Parameters affecting pharyngeal response to genioglossus stimulation in sleep apnoea. Eur Respir J 2011; 38:338–347.
• A comprehensive study evaluating multiple parameters that may influence response to genioglossus stimulation in OSA.
34.Oliven R, Tov N, Odeh M, et al. Interacting effects of genioglossus stimulation and mandibular advancement in sleep apnea. J Appl Physiol 2009; 106:1668–1673.
35.Oliven A, Odeh M, Geitini L, et al. Effect of coactivation of tongue protrusor and retractor muscles on pharyngeal lumen and airflow in sleep apnea. J Appl Physiol 2007; 103:1662–1668.
36.Schwartz AR, Benett ML, Smith PL, et al. Therapeutic electrical stimulation of the hypoglossal nerve in obstructive sleep apnea. Arch Otolaryngol Head Neck Surg 2001; 127:1216–1223.
37.Kezirian EJ, Boudewyns A, Eisele DW, et al. Electrical stimulation of the hypoglossal nerve in the treatment of obstructive sleep apnea. Sleep Med Rev 2010; 14:299–305.
• The most recent review describing the methodology and results of the phase I hypoglossus nerve stimulation study.
38.Eastwood PR, McEvoy RD, Wheatley J, et al. Hypoglossal nerve stimulation therapy to treat obstructive sleep apnea: interim feasibility trial results. Am J Respir Crit Care Med 2011; 183:A2726.
•• Most recent interim results of the HGNS stimulation device group. Three quarters of their patients, who had predominant hypopneas, experienced a more than 50% decline in the AHI. This improvement was associated with a significant symptomatic improvement.
39.Schwartz AR, Barnes M, Hillman D, et al. Acute effects of hypoglossal nervestimulation on airflow and arousal. Am J Respir Crit Care Med 2011; 183:A2728.
40.Badr MS, Oliven A, Maurer J, et al. Predictor of response for upper airway stimulation in obstructive sleep apnea. Am J Respir Crit Care Med 2011; 183:A2727.
•• Most recent interim results of the Inspire II stimulation device group. Analysis of an initial group of patients led to improved patients selection. In newly implanted patients, a decrease in AHI of more than 50%, to levels below 20, were found in eight out of nine patients.
41.Rodenstein D, Rombaux P, Dury B, et al. Unilateral targeted hypoglossal neurostimulation (THN) for the treatment of obstructive sleep apnea (OSA). Am J Respir Crit Care 2011; 183:A6383.
•• These investigators reported their results with an upgraded, refined electrode. Hypoglossus stimulation was applied continuously, during both inspiration and expiration. A significant reduction in AHI was obtained without apparent neuromuscular damage.
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest,
•• of outstanding interest.
Additional references related to this topic can also be found in the Current World Literature section in this issue (p. 485).
Acknowledgements
The present review was supported by the Binational USA-Israel Science Foundation (BSF) grant 2000234.
Conflicts of interest
There are no conflicts of interest.
Curr Opin Pulm Med. 2011;17(6):419-424. © 2011 Lippincott Williams & Wilkins