Poor quality, contaminated supplements
A new paper from Lauren Erland and Praveen Saxena raises concerns about the safety and quality of melatonin supplements. There was a variety of products purchased including tablets and liquids. Different lots of the same product were purchased, in order to examine variation in content lot-to-lot. The authors quantified the amount of melatonin, and also looked for the presence of serotonin. Their findings suggest that there’s little quality control in the products that were studied:
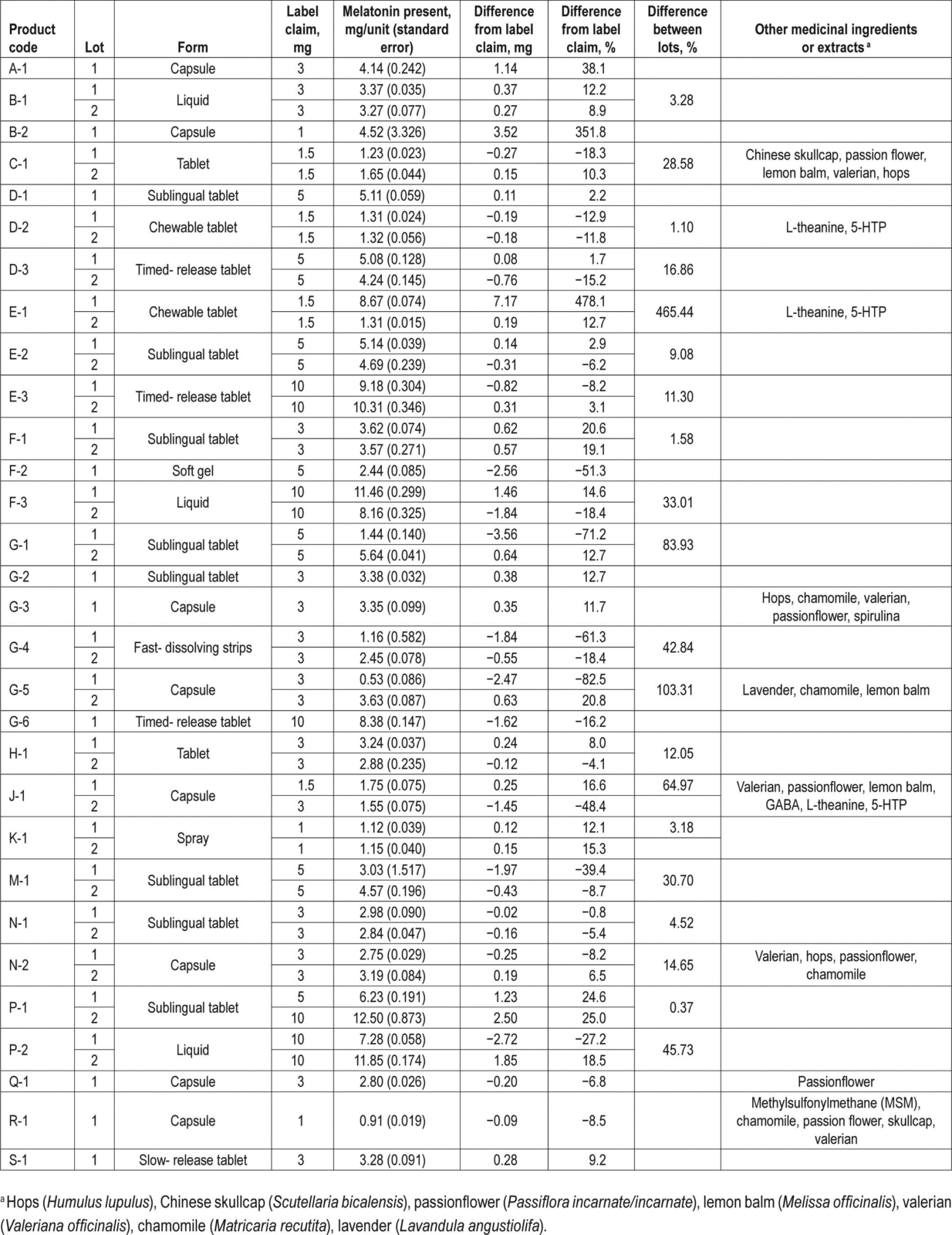
Melatonin content was found to be highly variable between samples and lots, with no pattern observed between brand, form of supplement, labelled value, or presence of other herbal extracts. The most variable sample, chewable tablet E1, showed a 478% increase from label claim containing almost 9 mg of melatonin, compared to the 1.5-mg label claim, though this was also highly variable between lots (465% difference). The supplement that showed the greatest decrease in melatonin content as compared to labelled values was the capsule G5 which contained lavender, chamomile, and lemon balm, with a decrease of 83%.
What’s on the label isn’t what’s in the bottle:
71% of products failed to meet label claims (per-dose content was expected to be +/- 10%).
Actual content ranged from 83% below labelled claim to 478% above labelled claim.
Lot-to-lot, product content varied by as much as 465%
Undeclared serotonin was found in 8 of the products. Serotonin is a precursor of melatonin and isn’t sold as a supplement. Serotonin syndrome is a well-documented reaction with some prescription medications. The presence of undeclared serotonin could potentially lead to serious adverse reactions.
Here’s Table 1, illustrating that manufacturers of melatonin do not appear to be producing consistent, high-quality products:
Buyer beware
These finding add to other studies that have found that dietary supplements may be manufactured to poor quality standards, with little assurance that what’s on the label is actually in the bottle. Not only does it appear difficult for consumers to identify a product of high quality, there’s no assurance that product content is even consistent, lot-to-lot. What would be really interesting would be to conduct the same analysis using product purchased in countries where melatonin is regulated as a drug. In doing so, we might obtain even more insight into manufacturing quality standards for drugs versus supplements. Given the drug approval standards in place in Canada, the United States, and around the world, I’d wager that those products would be found to be far more consistent in content and quality.
Until consumers and health professionals alike have confidence that dietary supplements are of uniform and consistent quality, and are free of undeclared contaminants, it’s not possible to use them safely, or to expect that they will deliver any predictable effects.The supplement marketplace continues to serve as a case study to those that think that looser regulation actually benefits consumers, or helps ensure better health care.
https://sciencebasedmedicine.org/melato ... he-bottle/
http://www.aasmnet.org/jcsm/ViewAbstract.aspx?pid=30950
Melatonin: What’s on the label isn’t in the bottle
Melatonin: What’s on the label isn’t in the bottle
Sheffey