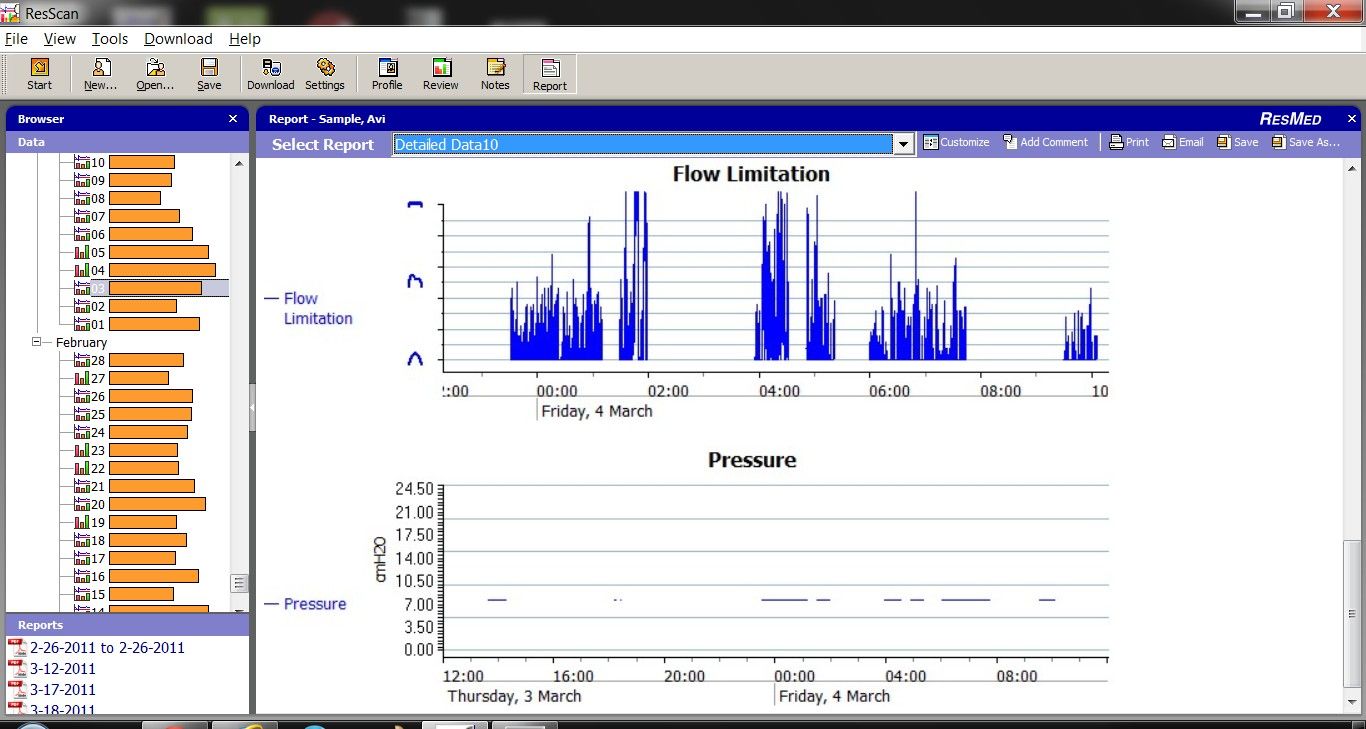
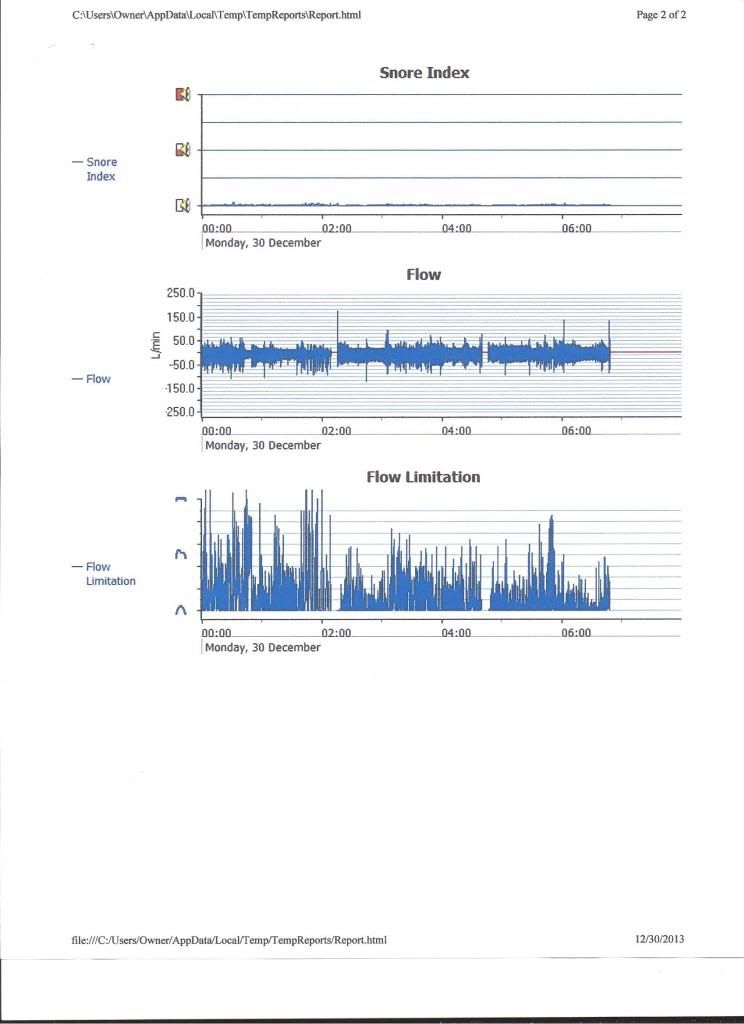
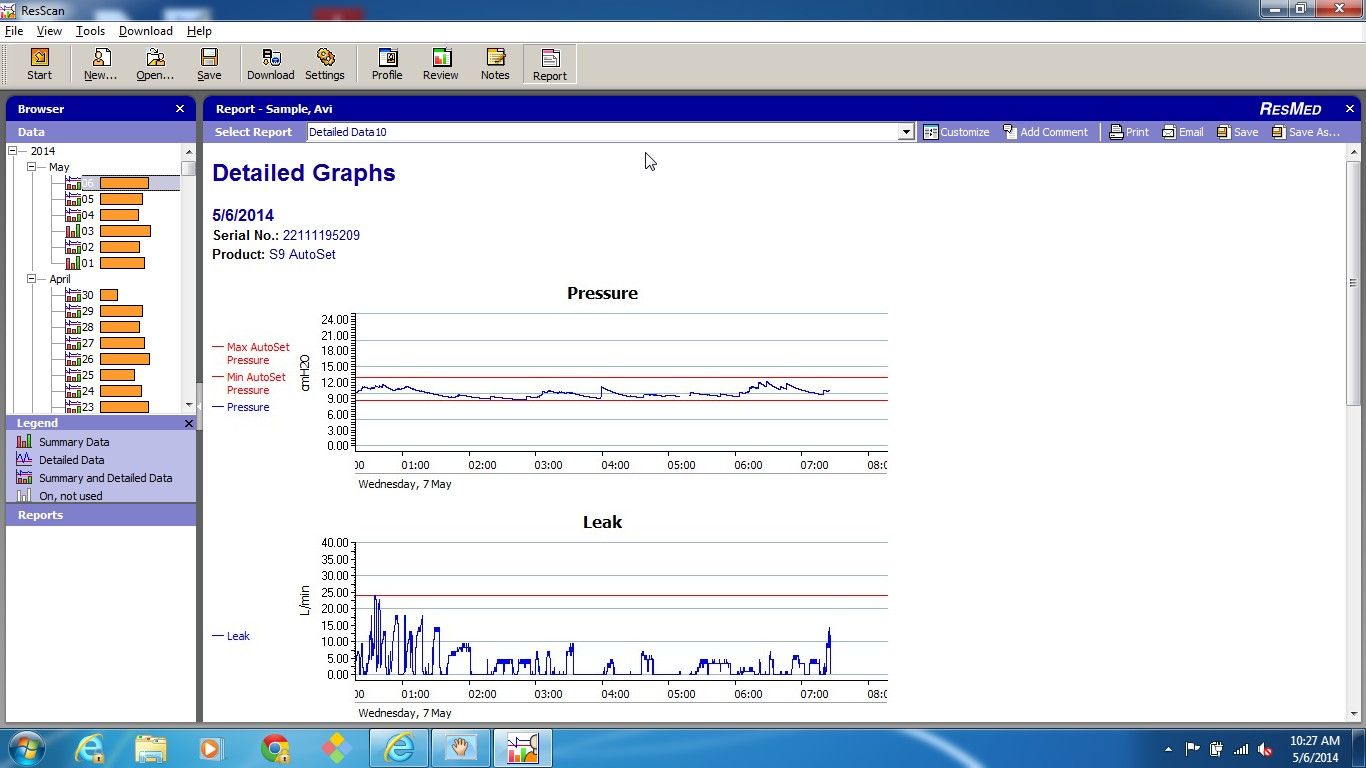
avi123 wrote:my Resmed S9 Autoset does provide a graph of Flow Limitation which also include also UARS. I think that I myself have some UAR syndrome.
This is a graph of the machine decided pressure during my treatment in APAP mode:
And this is how my Flow Limitation graph looks like:
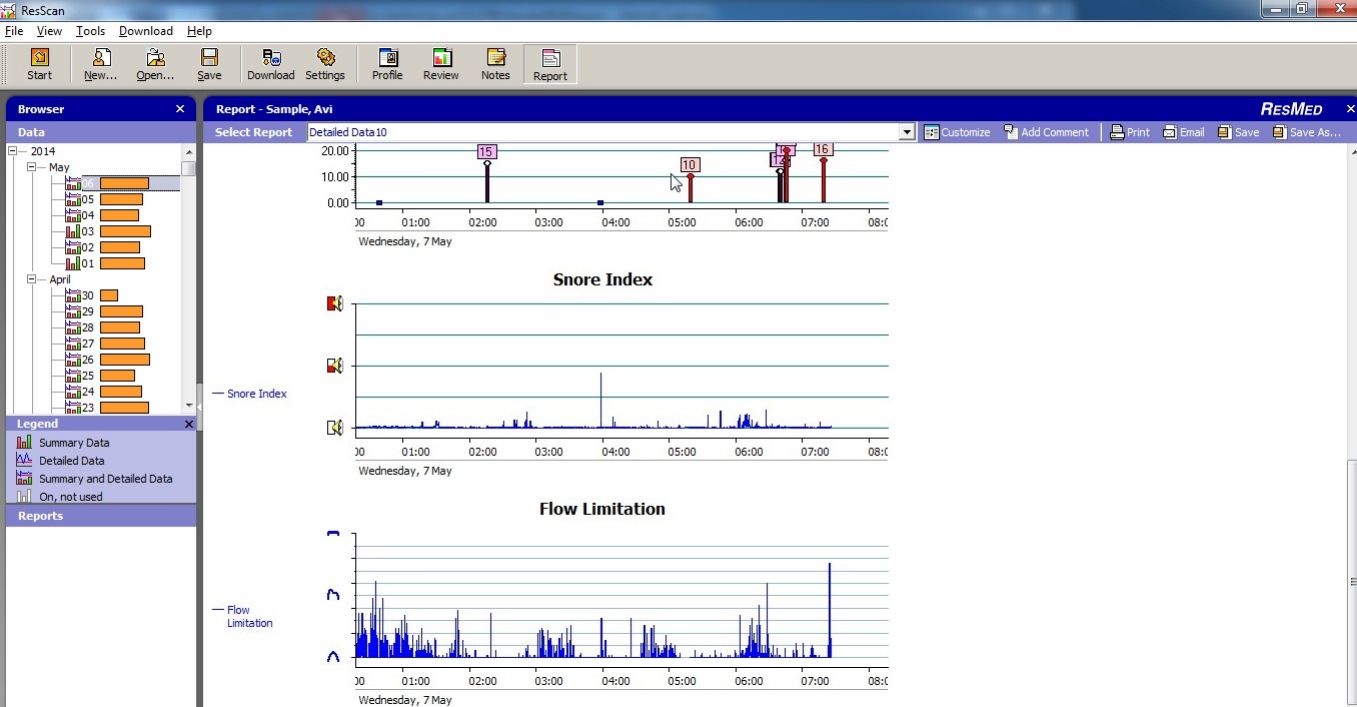
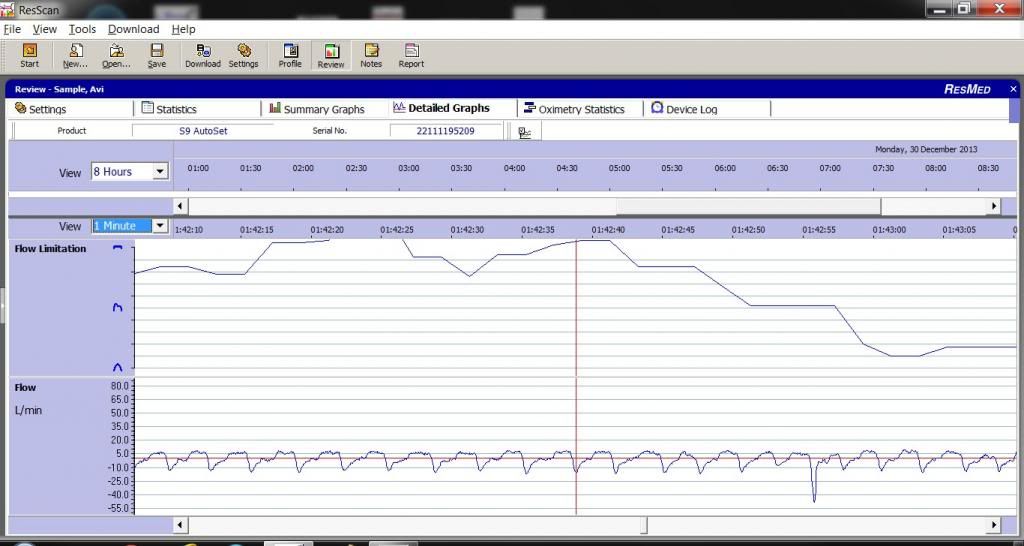
The machine looks at the shape of each respiration wave and decides how out of roundness the top shape of the curve is and gives it a weighting:
These are actual shapes of my respirations flow curves, and the calculated Flow Limitation graph, on top:
The following is the content of the article in the Chest website from 2011 authored by the following:
Olukayode Ogunrinde, MD; Herbert J. Yue, MD; and Christian Guilleminault, MD, BiolD
Dr. Ogunrinde is Clinical Fellow, Department of Sleep Medicine, Stanford University; Dr. Yue is Clinical Fellow, Department of Sleep Medicine, Stanford University; and Dr. Guilleminault is Professor, Division of Sleep Medicine, Stanford University Medical School, Redwood City, California.
Drs. Ogunrinde, Yue, and Guilleminault have disclosed no significant relationships with the companies/organizations whose products or services may be discussed within this chapter.
Objectives
1.Identify the demographics of previously described patients with upper airway resistance syndrome (UARS).
2.Describe the polysomnographic findings in UARS, including markers of increased respiratory effort.
3.Discuss the clinical symptoms of UARS and their link to functional somatic syndromes.
4.Describe the EEG findings during a respiratory event in UARS.
5.Identify the current treatment options for UARS, as well as the benefits and risks of each.
Key words: cyclical alternating pattern; sleep-disordered breathing; upper airway resistance syndrome
Abbreviations: CAP = cyclical alternating pattern; CPAP = continuous positive airway pressure; LAUP = laser-assisted uvuloplasty; MSLT = multiple sleep latency testing; NREM = nonrapid eye movement; OHS = obstructive hypopnea syndrome; OSAS = obstructive sleep apnea syndrome; Pes = esophageal pressure; REM = rapid eye movement; RERA = respiratory effort-related arousal; UARS = upper airway resistance syndrome
Professional Practice Gap
Over the past few years, numerous articles have been published that have increased our understanding of the features of upper airway resistance syndrome (UARS). UARS has been previously described as a distinct clinical syndrome, although there is ongoing controversy and some consider it to be part of a spectrum of sleep-disordered breathing that includes primary snoring, obstructive hypopnea syndrome (OHS), and obstructive sleep apnea syndrome (OSAS). However, patients with UARS present with different polysomnographic abnormalities and do not meet generally accepted criteria for either apneas or hypopneas. The lack of education about UARS in the medical community has allowed these patients to go undiagnosed and untreated. Increased research into UARS will help us to identify the optimal treatment for these patients, as well as to educate clinicians about this relatively under-recognized population.
Introduction
UARS is characterized by abnormal respiratory effort, nasal airflow limitation, absence of obstructive sleep apnea, minimal pulse oximetry fluctuation with oxygen saturation nadirs ≥92%, and frequent nocturnal arousals or reflex brainstem activation.1 It was first recognized in children in 1982, although the term UARS was not used until the first adult cases were reported in 1993.2,3 The incidence and prevalence of UARS has been systematically investigated in a recent São Paulo epidemiologic study4 (discussed below in “Epidemiologic Studies of UARS”). Prior to the São Paulo study, some have reported prevalence rates of 8% to 20% in the literature.5,6 We now know that this syndrome has recognizable clinical and polysomnographic characteristics that differ from those observed in patients with OHS/OSAS. UARS occurs in all age groups without any clear sex preferences, although some studies suggest that women may be at increased risk. UARS has the potential for significant impact on the daytime functioning and quality of life in untreated patients and there is growing evidence to suggest that symptoms are progressive without treatment.
Pathophysiology
Sleep-disordered breathing affects multiple aspects of the upper airway and respiratory tract, including the nasal passage, nasopharynx, oropharynx, hypopharynx, and supraglottic larynx. In these patients (primarily those with OSAS), multiple changes to the control of the upper airway have been described, including development of lesions in the soft tissue and changes in the upper airway dilator muscles, all in response to repetitive airway obstruction.7 Histologic studies have demonstrated significant abnormalities in biopsy samples taken from the palate and posterior pharynx. The most consistent finding has been evidence of a neurogenic lesion, with progressive abnormalities identical to those associated with a polyneuropathy.8,9 In patients with OSAS, poor responses to airway collapse or instability are often seen and thought to be related to blunting of the afferent upper airway receptors. In contrast to patients with OSAS, similar lesions have not been described in those with UARS. In a study of 45 subjects evenly divided into one group with OSAS, one group with UARS, and a control group, no significant sensory differences in palatal two-point discrimination were seen between control individuals and those with UARS.10 Of note, those with OSAS were observed to have significant decrements in their palatal two-point discrimination, consistent with the prior findings of neurogenic lesions. Other contributors to the pathophysiology of UARS include an increase in airway collapsibility owing to abnormal inspiratory and expiratory airflow dynamics.11,12 Increased nasal airflow resistance has also been thought to contribute to the breathing impairment seen in UARS, with frequent clinical findings of collapsible nasal valves, enlarged nasal turbinates, a deviated septum, or a combination of these findings in UARS patients.13
Epidemiologic Studies of UARS
The only known epidemiologic study of UARS has been one performed as part of an epidemiologic study of sleep disorders in the city of São Paulo, Brazil, in 2007. The São Paulo Sleep Epidemiologic Study is a population-based, cross-sectional study of sleep disturbances and their risk factors.4 Methodological details of the São Paulo Epidemiologic Sleep Study are published. A total of 1,042 volunteers underwent polysomnography at the Sleep Institute of the Federal University of São Paulo; the refusal rate was a very low 5.4%. The criteria used for scoring for UARS were those described by Bao and Guilleminault.14 Polysomnography was performed according to American Academy of Sleep Medicine guidelines.15 The prevalence of UARS for the total sample was 15.5%. It was higher in women (21.87% vs 15.19%) and also in people aged <40 years (31.04% vs 5.49%).
Clinical Features
Patients with UARS have symptoms similar to those seen in OSAS, although there are some distinct features. Much of the research performed has attempted to identify and describe a group of patients with significant daytime sleepiness and disrupted sleep, but without the other dominant clinical features seen in OSAS. Typical symptoms reported by patients with UARS include excessive daytime sleepiness, fatigue, difficulty concentrating, morning headaches, and unrefreshing sleep. There can be also be a significant impairment in daytime functioning; a recent study demonstrated that subjects with UARS performed worse than patients with obstructive sleep apnea hypopnea syndrome and normal control individuals on different aspects of the Psychomotor Vigilance Task.16 In a separate study, upwards of 30% of subjects with UARS had abnormal sleep-onset latency on the Maintenance of Wakefulness Test.17 Individuals with abnormal airway anatomy are at increased risk, including those with a decreased retrolingual space, narrow nasal passages, or a small neck circumference.6 Patients are typically not obese, with a mean BMI often <25 kg/m2.3 They are also usually younger than those in whom OSAS is diagnosed, with a mean age of approximately 38 years.3 Snoring is not a requisite symptom, with 10% to 15% or more of patients having never or only intermittently snored.3,18
Patients with UARS are also more likely to report symptoms of frequent nocturnal awakening with difficulty falling back to sleep.19 This is thought to be a potential reason for increased complaints of insomnia amongst patients with UARS, including sleep onset and sleep maintenance problems. In addition to difficulties with acute insomnia, patients with UARS also have an increased likelihood of carrying a diagnosis of chronic insomnia. Other notable complaints include parasomnias, especially sleepwalking, sleep talking, and sleep terrors.20 Patients may also have symptoms of abnormal autonomic function, including lightheadedness or dizziness on rising from a supine or sitting position, cold hands and feet, and low resting blood pressures (defined as a systolic BP <105 mm Hg with a diastolic BP <65 mm Hg). In a study of 400 patients with UARS, more than 20% met criteria for low BP, a significantly higher prevalence when compared with people who have OSAS (0.6%) or insomnia (0.9%).21 Interestingly, all subjects in the study had evidence of a small oral cavity on examination with a narrowed airway space dimension on cephalometric radiographs, consistent with other reports.22 Lastly, patients with UARS have increased rates of symptoms such as gastroesophageal reflux, muscular pain, diarrhea, abdominal pain, depression, and anxiety. This has led some authors to suggest a link between UARS and functional somatic syndromes, such as irritable bowel syndrome, chronic fatigue syndrome, and fibromyalgia. In a study of 75 subjects equally divided into three groups (UARS, mild to moderate OSAS, and severe OSAS), those with UARS were more likely to report symptoms of headache, irritable bowel symptoms, and sleep-initiation insomnia.23 Subjects with UARS were also more likely to have alpha intrusion during slow-wave sleep, a polysomnographic finding described in a number of fatigue syndromes.24 In children with UARS, symptoms consistent with attention deficit disorder or attention deficit hyperactivity disorder may be present, with behavioral changes leading to poor school performance.25 Table 1 lists symptoms associated with UARS.
--------------------------------------------------------------------------------
Tables and other illustrations are not included
Table 1—Clinical Features Associated With UARS
Daytime symptoms Excessive daytime sleepiness
Fatigue
Morning headaches
Myalgias
Difficulty concentrating
Sleep disturbances Frequent nocturnal awakenings
Difficulties initiating sleep
Insomnia
Bruxism
Restless leg syndrome
Unrefreshing sleep
Autonomic nervous system Hypotension
Orthostasis
Cold hands and feet
Functional somatic syndrome associations Depression
Anxiety
Chronic fatigue syndrome
Irritable bowel syndrome
Fibromyalgia
Polysomnographic abnormalities Increased RERAs
Increased nocturnal arousals
Increased CAP rate
Alpha intrusion during sleep
--------------------------------------------------------------------------------
Diagnosis
Patients with UARS can present with a constellation of generalized and/or specific symptoms and a clinical examination often demonstrates craniofacial abnormalities. As noted previously, many of the prior difficulties in making a diagnosis of UARS arose because these patients do not meet polysomnographic criteria for OSAS. In the original description of UARS back in 1991, repetitive increases in upper airway resistance associated with arousals were observed in 15 subjects, defined as an increase in the peak inspiratory esophageal pressure that immediately preceded a transient arousal.3 A diagnosis of UARS requires patient symptoms, evidence of upper airway narrowing or craniofacial abnormalities, and appropriate associated polysomnographic findings. When an overnight polysomnogram is performed on a patient with UARS, there are no apneas with a hypopnea index <5, the oxygen saturation is usually >92%, and there is evidence of respiratory effort-related arousals (RERAs). Flow limitation can also be present, identified as an abnormal contour in the nasal pressure transducer signal waveform.3,14 Other techniques have been used to detect the subtle changes in respiration, such as the oral/nasal thermistor, pneumotachograph, quantitative respiratory plethysmography, and nasal cannula/pressure transducer, although measurement of esophageal pressure (Pes), or esophageal manometry, remains the gold standard.26,27
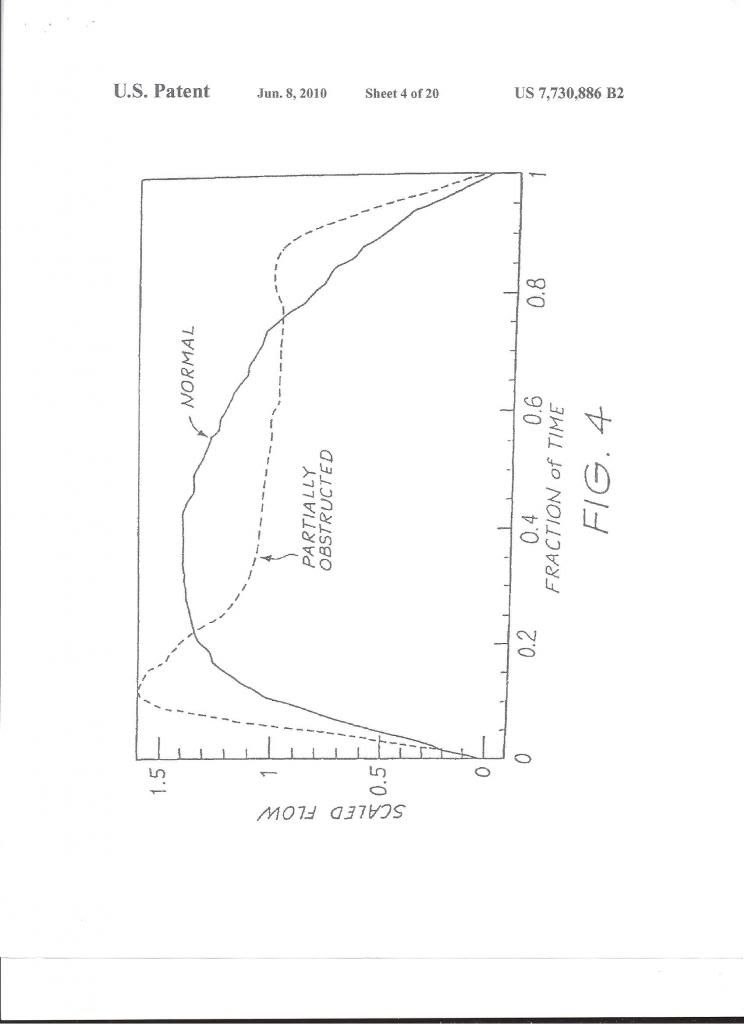
Typically, a pediatric feeding tube is used in the measurements of esophageal pressure during sleep. A number of characteristic Pes patterns associated with RERAs have been described.28,29 Pes crescendo (Fig 1) is a progressive increase in negative peak inspiratory pressure with each breath that terminates with an EEG arousal, typically a burst of alpha or delta activity. Other changes include sustained continuous respiratory effort; the Pes tracing shows a stable and persistently abnormal negative peak inspiratory pressure, different from the baseline pressure and present for more than four breaths. In both instances, the increase in respiratory effort is not usually associated with oxyhemoglobin desaturation and there may be a flattening in the nasal pressure transducer waveform, consistent with flow limitation (Figs 2, 3). The termination of both Pes crescendo and flow limitation is called Pes reversal, which is a decrease in respiratory effort indicated by a less negative peak inspiratory pressure, often without an associated EEG arousal (Fig 4). Heart rate variability is often seen during events of increased respiratory effort. Patients with UARS may also have more negative peak inspiratory pressure during nonrapid eye movement (NREM) sleep, especially slow-wave sleep, as compared with rapid eye movement sleep.30 Patients with UARS have also been described to have less NREM (both stage 1 and stage 2) sleep and more slow-wave sleep when compared with other patients with sleep-disordered breathing, although no significant difference in arousal threshold was seen.31
--------------------------------------------------------------------------------
Figure 1. Pes crescendo. Note the esophageal pressure recording at the bottom of the tracing.
L13Fig2
Figure 2. Flow limitation on a polysomnogram. Note the association between CAP, with phase A2 and A345 indicative of sleep disruption, and the continuous flow limitation seen on the nasal cannula recording (duration of sample, 5 min).
--------------------------------------------------------------------------------
L13Fig3
Figure 3. Flow limitation on polysomnogram monitored at slow speed (duration of sample, 30 s) Note the abnormal shape of the nasal cannula pressure transducer flow curve, with disappearance of the normal “round” curve with each breath. Note also the downward movement of the finger plethysmography (finger PPG) over-curve indicating sympathetic brainstem activation. Simultaneously, the EEG (right side of figure) changes to lower amplitude and faster frequency, indicative of a short arousal response.
--------------------------------------------------------------------------------
L13Fig4
Figure 4. Pes reversal. Note the esophageal pressure recording at the bottom of the tracing.
--------------------------------------------------------------------------------
Patients with UARS may also have characteristic changes in their EEG patterns during sleep. As noted previously, prior studies have shown that subjects with UARS have increased amounts of alpha frequency on EEG when compared with patients who have either OSAS or OHS.32,33 Other investigations using power spectrum EEG analysis have demonstrated that UARS patients have more theta and low alpha powers (7 to 9 Hz) during NREM sleep and more delta power during rapid eye movement sleep.29 Although an arousal is frequently seen following termination of an event associated with increased respiratory effort (ie, Pes crescendo or continuous increased respiratory effort), studies have shown that Pes reversal can occur in the absence of any clear arousal. In a study of 15 patients with UARS, a spectral analysis was performed on EEG data from central leads. The authors noted EEG changes in association with Pes events, including an increase in delta frequency activity prior to Pes reversal, and with increases in other frequencies after the event, suggesting that significant EEG changes are occurring with Pes events, even when an arousal is not visible.29
Lastly, patients with UARS have evidence of increased rates of the cyclical alternating pattern (CAP) on EEG. CAPs are a well-described pattern of NREM sleep, defined as electrocortical events that are distinct from background EEG activity and occur with a periodicity.34 An increase in CAP frequency is generally associated with sleep instability/disturbance and CNS hyperactivity and has been observed in a number of conditions, including sleep-disordered breathing, restless leg syndrome, and insomnia.31 In a study of 30 patients with UARS, increased CAP rates were seen in addition to more EEG arousals and higher markers of sleep disruption and transition (A2 and A3 indices of CAP).35 More importantly, the CAP rate was correlated with daytime symptoms of sleepiness and fatigue. This finding suggests that normalization of the breathing events in patients with UARS/sleep-disordered breathing may not be sufficient to fully treat their daytime symptoms and that addressing EEG abnormalities is important as well.
Treatment
The optimal treatment for patients with UARS is not currently known. Continuous positive airway pressure (CPAP) has been quite useful in the treatment of sleep-disordered breathing and there are some notable positive results in CPAP treatment of UARS. In a study of 15 heavy snorers with clinical evidence of UARS, treatment with nasal CPAP was associated with decreases in observed nocturnal arousals on polysomnography and decreases in mean sleep latency times on multiple sleep latency testing (MSLT) after several nights of treatment.3,36 A follow-up study of 15 subjects (in the original description of UARS) with daytime sleepiness and fatigue and who had undergone a therapeutic trial of positive pressure therapy reported similar findings.3 After treatment with approximately a month of nasal CPAP, significant improvements were seen in mean sleep latency times on MSLT (5.3 minutes vs 13.5 minutes), Pes nadir pressure (–33.1 cm H2O vs –5.3 cm H2O), amount of slow-wave sleep (1.2% vs 9.7%), and EEG arousals (31.3 vs 7.9 events/hour of sleep). Along with an improvement in sleep latency times on MSLT, there were subjective reports of improved daytime symptoms. Lastly, in a study of 130 postmenopausal women with chronic insomnia and evidence of UARS (n=62) or normal breathing (n=68), treatment with either nasal turbinectomy or nasal CPAP was associated with improvements in subjective reports of sleep quality as measured with a visual analog scale as well as mean sleep latency times on polysomnography.19 Despite the growing body of evidence supporting the use of positive pressure therapy for UARS patients, it remains difficult to obtain therapy. In a follow-up study of more than 90 patients conducted 4 to 5 years after the initial diagnosis of UARS was made, none of the subjects were receiving CPAP treatment; the main rationale given was that their insurance provider declined to provide the necessary equipment.1 Formal follow-up clinical evaluations of these patients noted significant worsening in their sleep-related complaints, with increased reports of fatigue, insomnia, and depressive mood. More disturbingly, prescriptions for hypnotics, stimulants, and antidepressants increased more than fivefold.
Other interventions, such as surgery or oral appliances, have also been used with some success in the treatment of patients with UARS. Procedures such as uvulopalatopharyngoplasty, laser-assisted uvuloplasty (LAUP), septoplasty with turbinate reduction, genioglossus advancement, and radiofrequency ablation of the palate have all been described in the literature.37-40 A study of LAUP in nine patients with UARS who underwent uvulopalatopharyngoplasty (n=2), multilevel pharyngeal surgery (n=1), or LAUP (n=6) reported improvements in subjective daytime sleepiness as measured with Epworth Sleepiness Scale scores.37 In the two patients for whom postoperative polysomnographic data was available, significant improvements in Pes nadir pressures were seen. But patients had several interventions and it is difficult to assess which one was successful. A study of 14 patients with UARS who underwent radiofrequency ablation of the palate also reported improvement in subjective sleepiness, with concurrent improvements in Pes nadir levels and reports of snoring.40 However, prior reviews of the available literature have noted that many of the studies evaluated small numbers of patients, consisted of uncontrolled case reports or series without clear characterization of the subjects enrolled, and had no consistent end points for an adequate evaluation of efficacy.39 Further investigation is required to determine the specific role for surgical intervention in these patients. Other authors have also reported successful treatment of UARS with use of oral appliances, although these studies suffer from the same limitations as the surgical literature.41 In children, orthodontic approaches, such as maxillary distraction or use of expanders, have also shown promising results.42
Conclusion
Although UARS has a symptomatology close to the one seen in patients with OSAS, there are distinct clinical differences between the two syndromes. In clinical studies, it is seen more in younger, slim subjects and in premenopausal women; it is more commonly associated with an increase in vagal tone during sleep than with sympathetic hyperactivity (as seen in association with apnea and hypopnea and oxygen desaturation).21 Can individuals with UARS become patients with OSAS? Guilleminault and colleagues1 suggested that weight increase (with development of a chest-bellow problem related to abdominal obesity) and the association of the supine position and sleep (leading to a restrictive impairment and secondary oxygen saturation drop and sympathetic hyperactivity) will lead to passage from one presentation to another with different complications; but more data are needed from additional systematic, longitudinal studies.1 UARS is underdiagnosed owing to unfamiliarity with the syndrome and the lack of polysomnographic criteria for either hypopneas or apneas that are associated with other types of sleep-disordered breathing. The advent of use of an esophageal catheter for esophageal pressure measurement (Pes) has allowed clinicians to more clearly identify patients with UARS. Although Pes measurement is the most sensitive method available to detect the abnormal respiratory events in UARS, it has not been used widely for several reasons, including lack of clinician experience and patient reports of discomfort. Usage of the nasal cannula pressure transducer allows recognition of flow limitation.43 But guidelines on how to tabulate the amount of flow limitation during total sleep time are lacking. Patients with UARS have significant impairment in their daytime functioning, with reports of sleepiness, fatigue, and sleep disruption. A follow-up study of these patients has shown that they often go untreated and experience progressive worsening of their symptoms. Among those patients who have been treated, typically with CPAP therapy, many have experienced symptomatic improvement. The current fund of knowledge regarding UARS has been growing, and we are beginning to understand the underlying pathophysiology.
--------------------------------------------------------------------------------
References
1.Guilleminault C, Kirisoglu C, Poyares D, et al. Upper airway resistance syndrome: a long-term outcome study. Journal of Psychiatr Res. 2006;40(3):273-279.
2.Guilleminault C, Winkle R, Korobkin R, Simmons B. Children and nocturnal snoring: evaluation of the effects of sleep related respiratory resistive load and daytime functioning. Eur J Pediatr. 1982;139(3):165-171.
3.Guilleminault C, Stoohs R, Clerk A, Cetel M, Maistros P. A cause of daytime sleepiness: the upper airway resistance syndrome. Chest. 1993;104(3):781-787.
4.Santos-Silva R, Tufik S, Conway SG, Taddei JA, Bittencourt LR. São Paulo Epidemiologic Sleep Study: rationale, design, sampling, and procedures. Sleep Med. 2009;10(6):679-685.
5.Chervin RD, Aldrich MS. Effects of esophageal pressure monitoring on sleep architecture. Am J Respir Crit Care Med. 1997;156(3 Pt 1):881-885.
6.Guilleminault C, Stoohs R, Kim Y, Chervin R, Black J, Clerk A. Upper airway sleep-disordered breathing in women. Ann Intern Med. 1995;122(7):493-501.
7.Petrof B, Hendricks JC, Pack AI. Does upper airway muscle injury trigger a vicious cycle in obstructive sleep apnea? A hypothesis. Sleep. 1996;19(6):465-471.
8.Edström L, Larsson H, Larsson L. Neurogenic effects on the palatopharyngeal muscle in patients with obstructive sleep apnoea: a muscle biopsy study. J Neurol Neurosurg Psychiatry. 1992;55(10):916-920.
9.Friberg D, Ansved T, Borg K, et al. Histological indications of a progressive snorers disease in an upper airway muscle. Am J Respir Crit Care Med. 1998;157(2):586-593.
10.Guilleminault C, Li K, Chen NH, et al. Two-point palatal discrimination in patients with upper airway resistance syndrome, obstructive sleep apnea syndrome, and normal control subjects. Chest. 2002;122(3):886-870.
11.Gold AR, Marcus CL, Dipalo F, Gold MS. Upper airway collapsibility during sleep in upper airway resistance syndrome. Chest. 2002; 121(5):1531-1540.
12.Gold AR, Dipalo F, Gold MS, Broderick J. Inspiratory airflow dynamics during sleep in women with fibromyalgia. Sleep. 2004;27(3):459-466.
13.Chen W, Kushida CA. Nasal obstruction in sleep-disordered breathing. Otolaryngol Clin North Am. 2003;36(3):437-460.
14.Bao G, Guilleminault C. Upper airway resistance syndrome—one decade later. Curr Opin Pulm Med. 2004;10(6):461-467.
15.Iber C, Ancoli-Israel S, Chesson AL, Quan SF. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Westchester, IL: American Academy of Sleep Medicine; 2007.
16.Stoohs R, Philip P, Andries D, Finlayson EV, Guilleminault C. Reaction time performance in upper airway resistance syndrome versus obstructive sleep apnea syndrome. Sleep Med. 2009;10(9):1000-1004.
17.Powers CR, Frey WC. Maintenance of wakefulness test in military personnel with upper airway resistance syndrome and mild to moderate obstructive sleep apnea. Sleep Breath. 2009;13(3):253-258.
18.Kristo DA, Lettieri CJ, Andrada T, Taylor Y, Eliasson AH. Silent upper airway resistance syndrome: prevalence in a mixed military population. Chest. 2005;127(5):1654-1657.
19.Guilleminault C, Palombini L, Poyares D, et al. Chronic insomnia, premenopausal women, and sleep disordered breathing: part 2. Comparison of nondrug treatment trials in normal breathing and UARS post menopausal women complaining of insomnia. J Psychosom Res. 2002;53(1):617-623.
20.Guilleminault C, Kirisoglu C, da Rosa AC, Lopes C, Chan A. Sleepwalking, a disorder of NREM sleep instability. Sleep Med. 2006;7(2):163-170.
21.Guilleminault C, Faul JL, Stoohs R. Sleep-disordered breathing and hypotension. Am J Respir Crit Care Med. 2001;164(7):1242-1247.
22.Ingman T, Nieminen T, Hurmerinta K. Cephalometric comparison of pharyngeal changes in subjects with upper airway resistance syndrome or obstructive sleep apnoea in upright and supine positions. Eur J Orthod. 2004;26(3):321-326.
23.Gold AR, Dipalo F, Gold MS, O’Hearn D. The symptoms and signs of upper airway resistance syndrome: a link to the functional somatic syndromes. Chest. 2003;123(1):87-95.
24.Manu P, Lane TJ, Matthews DA, Castriotta RJ, Watson RK, Abeles M. Alpha-delta sleep in patients with a chief complaint of chronic fatigue. South Med J. 1994;87(4):465-470.
25.Lewin DS, Di Pinto MD. Sleep disorders and ADHD: shared and common phenotypes. Sleep. 2004;27(2):188-189.
26.Ayappa I, Norman RG, Krieger AC, Rosen A, O’Malley RL, Rapoport DM. Noninvasive detection of respiratory effort-related arousals (RERAs) by a nasal cannula/pressure transducer system. Sleep. 2000;23(6):763-771.
27.Serebrisky D, Cordero R, Mandeli J, Kattan M, Lamm C. Assessment of inspiratory flow limitation in children with sleep-disordered breathing by a nasal cannula pressure transducer system. Pediatr Pulmonol. 2002;33(5):380-387.
28.Guilleminault C, Poyares D, Palombini L, Koester U, Pelin Z, Black J. Variability of respiratory effort in relation to sleep stages in normal controls and upper airway resistance syndrome patients. Sleep Med. 2001;2(5):397-405.
29.Black J, Guilleminault C, Colrain I, Carrillo O. Upper airway resistance syndrome: central electroencephalographic power and changes in breathing effort. Am J Respir Crit Care Med. 2000;162(2 Pt 1):406-411.
30.Guilleminault C, Poyares D. Arousal and upper airway resistance (UAR). Sleep Med. 2002;3 Suppl 2:S15-S20.
31.Stoohs R, Knaack L, Blum H, et al. Differences in clinical features of upper airway resistance syndrome, primary snoring, and obstructive sleep apnea/ hypopnea syndrome. Sleep Med. 2008;9(2):121-128.
32.Guilleminault C, Do Kim Y, Chowdhuri S, Horita M, Ohayon M, Kushida C. Sleep and daytime sleepiness in upper airway resistance syndrome compared to obstructive sleep apnoea syndrome. Eur Respir J. 2001;17(5):838-847.
33.Poyares D, Guilleminault C, Rosa A, Ohayon M, Koester U. Arousal, EEG spectral power and pulse transit time in UARS and mild OSAS subjects. Clin Neurophysiol. 2002;113(10):1598-1606.
34.Terzano MG, Parrino L. Origin and significance of the cyclic alternating pattern (CAP). Sleep Med Rev. 2000;4(1):101-123.
35.Guilleminault C, Lopes MC, Hagen CC, da Rosa A. The cyclic alternating pattern demonstrates increased sleep instability and correlates with fatigue and sleepiness in adults with upper airway resistance syndrome. Sleep. 2007;30(5):641-647.
36.Guilleminault C, Stoohs R, Duncan S. Snoring: daytime sleepiness in regular heavy snorers. Chest. 1991;99(1):40-48.
37.Newman JP, Clerk AA, Moore M, Utley DS, Terris DJ. Recognition and surgical management of the upper airway resistance syndrome. Laryngoscope. 1996;106(9 Pt 1):1089- 1093.
38.Utley DS, Shin EJ, Clerk AA, Terris DJ. A cost-effective and rational surgical approach to patients with snoring, upper airway resistance syndrome, or obstructive sleep apnea syndrome. Laryngoscope. 1997;107(6):726-734.
39.Pépin JL, Veale D, Mayer P, Bettega G, Wuyam B, Lévy P. Critical analysis of the results of surgery in the treatment of snoring, upper airway resistance syndrome (UARS), and obstructive sleep apnea (OSA). Sleep. 1996;19(9 suppl):S90-S100.
40.Powell NB, Riley RW, Troell RJ, Li K, Blumen MB, Guilleminault C. Radiofrequency volumetric tissue reduction of the palate in subjects with sleep-disordered breathing. Chest. 1998;113(5):1163-1174.
41.Loube DI, Andrada T, Shanmagum N, Singer MT. Successful treatment of upper airway resistance syndrome with an oral appliance. Sleep Breath. 1997;2(4):98-101.
42.Pirelli P, Saponara M, Guilleminault C. Rapid maxillary expansion in children with obstructive sleep apnea syndrome. Sleep. 2004;27(4):761-766.
43.Aittokallio T, Saaresranta T, Polo-Kantola P, Nevalainen O, Polo O. Analysis of inspiratory flow shapes in patients with partial upper-airway obstruction during sleep. Chest. 2001;119(1):337-344.
44.Terzano MG, Parrino L, Chervin R, et al. Atlas, rules and recording techniques for the scoring of the cyclical alternating pattern (CAP) in human sleep. Sleep Med. 2001;2(6):537-553